The Pediatric Sleep and Wellness Project is a prospective data collection project in partnership with the Loma Linda University Health Sleep Center. Our aim is to learn more about youth ages birth through 18 who are presenting to the Sleep Center for an overnight sleep study (polysomnography). Specifically, we want to describe the characteristics of these youth and their families and understand how different kinds of sleep problems are related to aspects of emotional and behavioral functioning, physical health, and family functioning. We hope that our findings will inform recommendations for the clinical care of youth with sleep problems.
THE PEDIATRIC HEALTH BEHAVIOR LAB
Tori Van Dyk, PhD
About Us

- Examine the ways in which mental and physical health are related in youth.
- Evaluate how positive changes in health behaviors such as sleep, diet, and physical activity are related to emotional and behavioral functioning and other physical health outcomes.
- Identify mechanisms, predictors, and intervention targets for negative physical and psychological outcomes in children and adolescents.
- Inform prevention, intervention, and public policy efforts to improve the lives of youth in our community.
Our Team
Alumni
Jada Hammond, PsyD
Alumni
Tiffany Vo, PsyD
Alumni
LLUCH Pediatric neurology Clinical Practicum


In collaboration with the LLUH Sleep Disorders Center and Loma Linda University Children’s Health (LLUCH) Division of Neurology, we offer practicum positions for Internal, External, and Pre-Internship Practicum Trainees. Trainees have the opportunity to work alongside pediatric neurologists in an integrated clinic setting providing behavioral medicine services for pediatric patients presenting with sleep or other neurological concerns (e.g., headache). The assessment and treatment of sleep problems and migraines in youth will make up the vast majority of the practicum experience. Trainees will have the opportunity to provide:
Integrated Clinic Evaluations: Trainees will complete evidenced-based evaluations of pediatric sleep concerns within the context of an integrated sleep medicine clinic. The trainee and physician will conjointly complete new patient evaluations and will provide joint diagnoses and treatment plans to patients and their families.
Behavioral Sleep Medicine Interventions: Trainees will provide youth and their families with evidenced-based behavioral sleep medicine interventions. These interventions will be provided within the context of the first new visit appointment following a thorough assessment (see above) and during follow-up visits, as needed. Behavioral sleep medicine interventions will be alongside sleep medicine recommendations (e.g., CPAP, medication, further assessment via PSG).
Behavioral Management of Pediatric Headache or Other Health Problems: Trainees will provide behavioral medicine services to assist patients in managing headaches and other health problems. These interventions will include cognitive behavioral therapy for pain management and interventions to target adherence and lifestyle changes.
Other Psychological Intervention Opportunities: Although trainees will primarily be intervening with concerns related to health and health behaviors (e.g., sleep problems, migraine), opportunities to manage general mental health concerns (e.g., anxiety, depression) will be available.
Research Opportunities: Opportunities to engage in clinically applied research may be available including data collection, entry, and analysis resulting in possibilities for research posters and publications.
Behavioral Sleep Medicine Recommendations
Research
Insufficient sleep affects about 70% of the adolescent population in the United States. Poor adolescent sleep in particular is linked to impaired academic performance, lower cognitive functioning, poor emotional regulation, and poor mental health. Treating sleep disturbance with exogenous melatonin has significantly increased over the past decade, as it is listed as the second-most used natural product within a child population by the National Institutes of Health. Further, adolescents 13 years of age or older are the most likely age group to be recommended melatonin for sleep difficulties, yet the acute effects of melatonin on sleep, daytime functioning, behavior, and mood are largely unknown.
MARS plans to fill this gap through a feasibility study that (a) focuses on typically developing adolescents aged 13-17, a neglected population in previous studies; (b) is experimental in nature, to allow for cause-effect inferences; and (c) measures multiple outcome domains, including both objective and subjective data, to maximize scientific yield. The investigative team includes expertise in pediatric neurology, health psychology, and sleep and are uniquely positioned to investigate the relationships between adolescent melatonin supplementation; sleep (i.e., actigraphy, sleep disturbance, fatigue, dim light melatonin onset); daytime functioning (i.e., mood, emotional regulation, behavior difficulties, physical activity); and any reported adverse effects.
Findings from this study will allow for preliminary causal conclusions about the potential benefits and risks of exogenous melatonin in typically developing youth with behavioral sleep concerns. Doing so will provide much needed empirically supported guidance to medical professionals and caregivers when considering melatonin supplementation as a treatment for adolescent sleep difficulties.
Participant recruitment for this study has been completed.
The Study to Investigate the Effect of Sleep Treatment for families of children with Autism (SIESTA) is a research project that compares two brief interventions aimed at improving child health and behavior outcomes of youth with autism. One intervention is focused on improving child sleep while the other is focused on improving child diet and physical activity. We are also interested in learning how parenting stress and child behavior are related to child health and sleep both before and after the intervention.
For more information about this study and information about participation please see this link: https://www.siestaproject.net/.
Posters
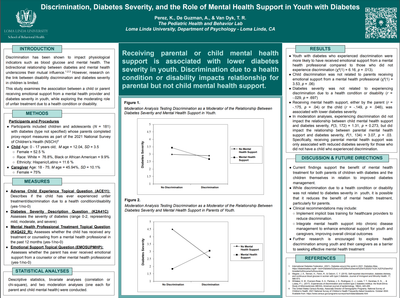
Perez, K., De Guzman, A., & Van Dyk, T.R. (August 8-10, 2024).
Read MoreWong, M.K., & Van Dyk, T.R. (August 8-10, 2024).
Read MorePerez, K., Van Dyk, T. (2024, April).
Read MoreEsquibel, K., Moeller, M., Morales, N., Dobalian, D., Van Dyk, T. (April 2024).
Read MoreMoeller, M., Esquibel, K., Morales, N., Van Dyk, T.R. (2024, April 25-27).
Read MoreMorales, N., Mazzone, E., Esquibel, K., Moeller, M., Van Dyk, T.R. (2024, April 25-27). Poster presented at the Society of Pediatric Psychology Annual Conference, New Orleans, Louisiana.
Read MoreDe Guzman, A., Perez, K., Van Dyk, T. (2023, April).
Read MoreTriplett, O. M., Mazzone, E., Morales, N., Van Dyk, T. R. (2023, April 27-30). Poster presented at the Western Psychological Association Convention, Riverside, California.
Read MoreMorales, N., Mazzone, E., Van Dyk, T. R. (2023, March 30-April 1). Poster presented at the Society of Pediatric Psychology Annual Conference, Chicago, Illinois.
Read MoreFeghali, P., Iwamoto, B., Triplett, O, Van Dyk, T. (2021, April)
Read MoreStudent Awards
APA Student Diversity Poster/Paper Award from Division 54. American Psychological Association Annual Conference, 2024
Read MoreAmerican Psychological Association Annual Conference, 2024
Read MorePublications
- DiFrancesco, M. W., Van Dyk, T. R., Altaye, M., Drummond, S. P., & Beebe, D. W. (2019). Network-based responses to the psychomotor vigilance task during lapses in adolescents after short and extended sleep. Scientific Reports, 9, 13913.
- Durracio, K. M., Krietsch, K., Chardon, M. L., Van Dyk, T. R., & Beebe., D.W. (2019). Poor sleep and adolescent obesity risk: A narrative review of potential mechanisms. Adolescent Health, Medicine and Therapeutics, 10, 117-130.
- Van Dyk, T.R., Zhang, N., Combs, A., Howarth, T., Whitacre, C., McAlister, S., & Beebe, D. (2019). Feasibility and impact on daytime sleepiness of an experimental protocol inducing variable sleep duration in adolescents. PLoS One, 14, e0218894.
- Van Dyk, T.R., Becker, S. P., & Byars, K. C. (2019). Rates of mental health symptoms and associations with self-reported sleep quality and sleep hygiene in adolescents presenting for insomnia treatment. Journal of Clinical Sleep Medicine, 15, 1433-1442.
- Van Dyk, T.R., Becker, S. P., & Byars, K. C. (2018). Psychopathology symptoms in preschool and school age youth presenting to insomnia evaluation: Prevalence and predictors of sleep disruption. Behavioral Sleep Medicine, DOI: 10.1080/15402002.2018.1518224.
- Van Dyk, T. R., Krietsch, K., Saelens, B. E., Whitacre, C., McAlister, S., & Beebe, D. W. (2018). Inducing more sleep on school nights reduces sedentary behavior without affecting physical activity in short-sleeping adolescents. Sleep Medicine, 47, 7-10.
- Van Dyk, T. R., Zhang, N., Catlin, P. A., Cornist, K., McAlister, S., Whitacre, C., & Beebe, D. W. (2017). Feasibility and emotional impact of experimentally extending sleep in short-sleeping adolescents. SLEEP, https://academic.oup.com/sleep/article/40/9/zsx123/3982412.
- Becker, S. P., Sidol, C. A., Van Dyk, T. R., Epstein, J. N., & Beebe, D. W. (2017). Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: A systematic review. Sleep Medicine Reviews, 34, 94-121.
- Nelson, T.D., Kidwell, K.M., Lundahl, A., & Van Dyk, T.R. (2016). LayHealth+: An innovative model for increasing access to high-quality health promotion services for Hispanic families. In K. Dombrowski & K. G. Carrasco (Eds.), Reducing Health Disparities: Research updates from the field. (pp. 91-111). Lincoln, NE: Syron Design Academic Publishing.
- Van Dyk, T. R., Thompson, R. W., & Nelson, T. D. (2016). Daily bidirectional relationships between sleep and mental health symptoms in youth with emotional and behavioral problems. Journal of Pediatric Psychology, 41, 983-992.
- Nelson, T. D., Van Dyk, T. R., McGinnis, C., Nguyen, A. V., & Long, S. K. (2016). Brief sleep intervention to enhance behavioral parent training for noncompliance: Preliminary findings from a practice-based study. Clinical Practice in Pediatric Psychology, 4, 176-187.
Contact

Tori Van Dyk, PhD
tvandyk@llu.edu
office (909) 558-7412
**Since email communication isn’t inherently encrypted and secure, please abstain from including in your message to us any details about your child’s medical condition or treatment. It is our policy and duty as a healthcare organization to not use email when sharing confidential information, so we will respond to your inquiry via secure methods only.